
Types of Clinical Trial Design
From the Desk of Dr. Danielle Meadows
Vice President of Research Programs & Operations

Designing a clinical trial is a complex and intricate process that can have a critical impact on the success or failure of the trial, so I want to jump back to the first stage of the research process for this month’s deeper dive.

In my view, clinical trial design consists of a couple of components: clinical trial structure and how the trial is conducted. For now, I’ll put more focus on clinical trial structure and delve into how the trial is conducted at other times.
The Heart of the Matter
- Clinical trial design consists of clinical trial structure and how the study is conducted, and it can have a significant impact on the success or failure of the trial.
- There are many types of clinical trial structures, but four of the main ones with relevance for ME/CFS are: parallel, crossover, adaptive, and factorial.
- Each structure addresses different objectives, including the ability to test multiple treatments and to use each participant as their own control.
- OMF’s Life Improvement Trial has a factorial clinical trial structure, allowing for testing the interaction of pyridostigmine and low-dose naltrexone.

Support our May Momentum Campaign! Please consider making a donation today to help us continue the momentum for OMF’s critical research.
Your gift—big or small—will help us expand our clinical trial network, allowing us to test more treatments and accelerate progress for people with ME/CFS and Long COVID.
Types of Clinical Trial Design
Clinical trials are critical for identifying and implementing effective treatments for diseases like ME/CFS and Long COVID. They serve as a controlled, systematic way to understand if and how a treatment works.
Clinical trial structures
Clinical trial structure refers to the component of trial design that details which treatments are given to which participant groups and at what time. There are many types of clinical trial structures, but some of the main ones are:
- Parallel: A parallel design is the most common kind of structure meant for treatment comparison. In this structure, treatment and control groups are tested simultaneously.
- Factorial: A factorial design is an extension of a parallel design. In this structure, treatments are still tested simultaneously, but it allows for testing the interaction of the treatments.
- Crossover: A crossover design is another type of comparison structure in which participants serve as their own control. In this structure, the order in which treatments are received is randomized, but each participant receives both the treatment and control.
- Adaptive: In an adaptive design, there is a planned opportunity to modify one or more aspects of the study design or hypotheses based on an interim analysis of data. For example, the probability of being assigned to a treatment that has a higher chance of success might be changed based on initial positive results.
Some clinical trial structures can be hard to imagine based on a description, so if you prefer a graphical depiction of the structures, check out the following.
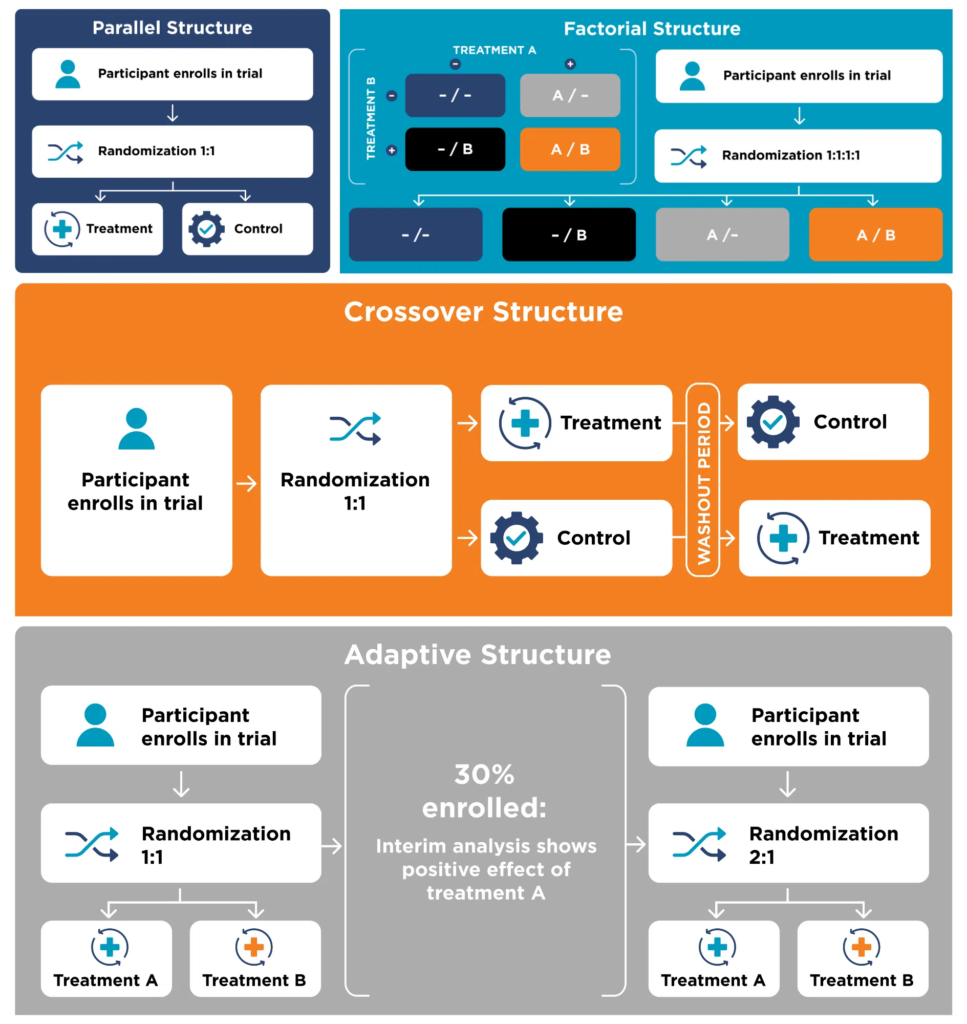
Each of these four types of clinical trial structure has some relevance for the ME/CFS research field, addressing different objectives. The parallel structure is a simple design that’s ideal for a “does it work?” kind of treatment comparison. The factorial structure, as the description above indicates, is best suited for studying multiple treatments, both individually and in combination. For a disease as complex and multi-systemic as ME/CFS, evaluating multiple treatments is needed. As ME/CFS afflicts a heterogeneous population, a crossover structure may be helpful. Having each participant serve as their own control can remove some of the confounding factors that influence results. Finally, an adaptive structure is attractive for use in ME/CFS because participants are more likely to receive an effective treatment. While this structure makes interpreting the results of the trial significantly more complicated, it may be worthwhile in some cases.
As a practical example, since Open Medicine Foundation’s Life Improvement Trial (LIFT) is testing pyridostigmine, low-dose naltrexone, and the combination of the two drugs, it uses a factorial clinical trial structure. For more information about the LIFT, you can read the protocol paper here.
Why is clinical trial design so important?
The design of a clinical trial can impact the success or failure of the trial, regardless of whether the treatment itself is effective or not. Therefore, it’s critical to select a clinical trial structure that is best suited for the objective of that particular study.
On top of needing to choose the structure that fits the trial objective, other components of clinical trial design are critically important for the participant experience. There are aspects of this that will be covered at other times (e.g., digital health and participant optionality), but the clinical trial structure will also impact the participant experience. Ultimately, it’s important to balance the scientific objectives and what’s best for the patients.